Here is some additional information along with references for your consideration.
The hypothetical equilibrium condition for Henry’s Law means that the ratio of the number of gas molecules of a specific gas being absorbed by a liquid surface versus the number of molecules of the same gas being emitted from that liquid surface is a constant for the given temperature of the surface. Unlike total pressure of mixed gases where at equilibrium the amount of total gas emitted equals amount of total gas absorbed, instead, Henry’s Law applies to partial pressure of a single specific gas, wherein, the ratio absorption vs emission of the single gas is the Henry’s Law partition ratio, or coefficient. Absorption and emission are occurring simultaneously, but not at the same rate. The absorption rate of a specific gas like CO2 into water surface can be increasing while simultaneously the rate of emission of CO2 from the nearby water surface can also be increasing; the conditions causing these two opposing perturbations to the Henry’s Law partition ratio for CO2 gas and water are different in the gas matrix and the liquid matrix.
When CO2 is added to air by burning fossil fuels, other sources of CO2 are naturally reduced and/or sinks are naturally increased so that the Henry’s Law ratio is maintained for the surface local temperature. It is a dynamic equilibrium and the control knob for the gas concentration, if you will, is the Henry’s Law ratio at the local surface temperature. CO2 is not accumulating in the atmosphere as hundreds of scientists and millions of dollars spent on annual carbon budgets attempt to persuade. CO2 has been increasing slowly in recent years because sea surface temperatures (SST) have been increasing in recent years. If and when SSTs begin decreasing, the CO2 concentration in air will also begin declining, though this decline is slow due to the enormous (orders of magnitude) higher heat capacity of ocean compared to atmosphere. For now, slowly warming SST and rising CO2 are both good for life.
Warming and cooling of ocean are very slow processes requiring centuries or millennia due to the enormous heat capacity of the ocean. The causes of warming and cooling are beyond the scope of this email except to point out firmly that they are definitely not caused by human-produced CO2; this statement does not require refutation or denial of the so-called “greenhouse gas” theory, radiative emission theory, carbon budgeting, feedbacks, etc.
The Henry’s Law coefficient is usually defined as kH = ca/pg
Where:
- kH is the Henry’s Law constant, or coefficient or partition ratio and refers to standard conditions, T = temperature = 298.15 K = 25 degrees C, and total pressure is 1 atm.
- ca is the concentration of the unreacted solute trace gas in the liquid phase
- pg is the partial pressure of the same solute gas in the gas phase in continuous contact with the surface of the liquid phase.
This version of Henry’s Law above requires calculations and measurements in volume for pg.
One of the common problems encountered with Henry’s Law is its dependence on temperature. kH is a constant (or coefficient, or ratio) that varies with temperature. This is not a simple matter. Temperature is a co-dependent variable with many other natural variables, including CO2 concentration, water vapor concentration, etc. For example, one of the teachers for chromatography classes for the American Chemical Society, who was one of the founding fathers of gas chromatography, published errors of Henry’s Law constants derived for temperature ranges; he was not alone. No doubt my own writings in my blog have errors too. Derivation of Henry’s law constants as a function of temperature is based on solution of the Clapeyron or Van Hoff equations formulated for water-gas equilibrium. The Clausius – Clapeyron equation specifies the temperature dependence of vapor pressure at a phase transition of a single gas between two phases of matter. Great care is required with the units. I will not go through the math of this except to present it since we will not need it or use it here.
dln kH/dT = ∆Hdis/RgT2
Where:
- ∆Hdis is the enthalpy (or heat) of dissolution of the gas solute in water
- Rg is the gas constant
- T is temperature in Kelvin.
Henry’s Law constant for CO2 gas and water solution. (as given in reference 5 below)
kH(T) = k°H exp(d(ln(kH))/d(1/T) ((1/T) – 1/(298.15 K)))
k°H = Henry’s law constant for solubility in water at 298.15 K (mol/(kg*bar))
d(ln(kH))/d(1/T) = Temperature dependence constant (K)
For our purposes studying CO2, atmospheric gases and Henry’s Law, a different derivation of Henry’s Law is much more convenient. Rolf Sander provides this derivation. The Henry’s Law coefficient is derived in its dimensionless (or unitless) form as the quotient (or ratio) of the molar concentration of the unreacted solute gas dissolved in the liquid phase matrix divided by the molar concentration of the same solute gas in the gas phase matrix. It is dimensionless since the units drop out when dividing molar mass by molar mass. But, the derivation of Henry Law for its dependence on temperature is still difficult. Today fortunately we look up Henry’s coefficients in reference books or by online software and then carefully verify the units since these experiments have been done thousands of times. There is a different kH for each gas and liquid combination and each temperature.
Henry’s Law expert Rolf Sander provides further expansion of various derivations in section 4.2 of the IUPAC Recommendations 2021 paper with other detail on the other forms of Henry’s law for various purposes here: https://www.degruyter.com/document/doi/10.1515/pac-2020-0302/html and references (4) and (5) below.
These two equations for Henry’s law, the partial pressure version above and the dimensionless version below, are related through the Ideal Gas Law:
kH = kH x RT
Where:
- R = the gas constant, which relates the energy scale in thermodynamics and physics to the temperature scale and the scale used for the amount of a substance such as moles:
- T = temperature in Kelvin.
The dimensionless version of Henry’s Law:
kH = ca/cg
Where:
- kH = the Henry’s Law coefficient
- ca = concentration of the gas in moles in the aqueous phase
- and cg is concentration of the same gas in the gas phase above the liquid/gas surface.
Generally, the dimensionless version is most convenient because the defacto NOAA-Scripps Mauna Loa “gold standard” CO2 data has been diligently measured and reported for many decades as micromoles of CO2 per mole of dry air. Micromoles of CO2 per mole of dry air is identical to ppm, parts per million by mass. This is a mass measurement, not a volume measurement, which is far more practical, precise and accurate for measuring trends in atmospheric gases since air always contains the gas water vapor, the quantity of water vapor in air is highly variable which causes the sample volume to be highly variable. Water vapor concentration in air is normally 10 to 100 times higher than the trace gases like CO2, methane (CH4), etc. Also, water vapor and CO2 have overlapping wavelengths of infrared light used in measurement instrumentation. These are reasons NOAA-Scripps dries their air samples (by freezing) and measures by mass instead of volume, i.e., micromoles of CO2 per moles of dry air which equals ppm.
Important note: ppm is not the same as ppmv. This is a common and large source of error in measuring net CO2 trends compared to the minor annual change in net global CO2 concentration.
Henry law coefficients are available online, having been measured thousands of times for thousands of combinations temperatures, liquid solvents and gas solutes. The concentration of CO2 in water is easily calculated from the gas concentration and the Henry’s coefficient, but the CO2 and H2CO3 (carbonic acid) concentrations are difficult or impossible to measure in water with acceptable accuracy and precision because sampling procedures affect the hydration reaction.
The gas-water surface interface can be the surface of a bubble at 3000 meters depth in ocean, or the surface of a raindrop in a cloud at 3000 meters altitude, lung tissue, leaf tissue, or dominantly the surface of the ocean.
In a mixture of gases, the flux of one gas back and forth across the surface does not affect the flux of a different gas until concentrations are very high. This was demonstrated by Adolph Fick, a physiologist, who studied air gas fluxes in lung tissues, a contemporary of William Henry, Thomas Graham, and John Dalton.
In nearly pure water like raindrops, the aqueous CO2 gas concentration is high and the H2CO3 (carbonic acid) concentration is high relative to the HCO3– (bicarbonate) concentration. Thus raindrops are measured slightly acid. Carbonic acid is a weak acid. But those raindrops also absorb other gases of nitrogen and sulfur (for example near an urban area or a refinery) which create strong acids in water. In contrast, in seawater HCO3– is the dominant species because there are very many more ionic species (sodium, calcium, etc.) dissolved in seawater which easily pull the first hydrogen ion away from the H2CO3. The concentration of calcium ion by itself is about 4 times as abundant in seawater surface as the combined concentrations of unreacted CO2 gas, bicarbonate ion, carbonate ion, and carbonic acid. The first dissociation constant for pulling away the first hydrogen atom from H2CO2 leaving HCO3– is very small and the reaction is nearly instant. So, dependent on the seawater conditions, HCO3– can be both a reaction product from the CO2 gas hydration reaction and also a reaction product from the dissociation reaction of H2CO3.
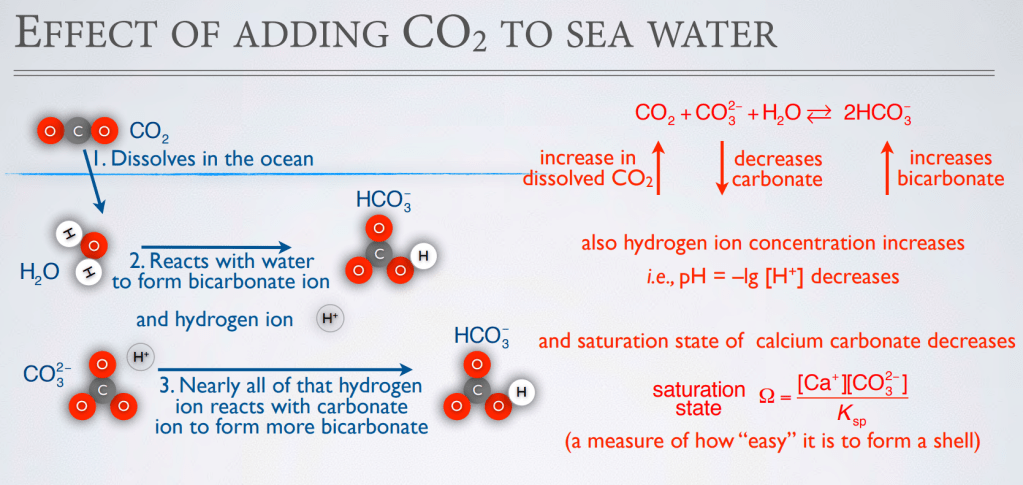

Graphics above from Andrew G. Dickson, Scripps Institution of Oceanography, UC San Diego, INTRODUCTION TO CO2 CHEMISTRY IN SEA WATER.(6)
There are many simultaneous phenomena occurring at these air/liquid surfaces. I have not touched on most of them. The most important quantitatively is the physical phase state reaction, wherein the change in partial pressure of the solute gas within the gas matrix causes a proportional change in the solubility of that unreacted solute gas in the liquid phase. When humans or any other source add CO2 to air, then the solubility of CO2 gas in water increases proportionally to absorb that CO2 gas. Double the partial pressure of CO2, then solubility of CO2 in water doubles. And vice versa.
The Henry’s Law ratio is a variable of surface temperature, not a variable of the amount of gas present, and not a variable of the source of the gas; it applies to all gas solutes in low concentrations with respect to the liquid solvents with which they are in continuous contact. The ratio does not apply to any portion of the gas solute which has reacted with the liquid solvent itself or has reacted with any component dissolved in the liquid solvent. Residence time, or half-life, or residual fraction, of the CO2 in air are not variables in the Henry Law phase-state equilibrium and play no role in net global CO2 concentration.
Next in importance is to understand that the CO2 hydration reaction (CO2 + H20 <-> H2CO3) is nearly instantly reversible, for example by a very minor increase in water temperature, or agitation of the water, or change in other variables. Then the first dissociation constant K1, which is dissociation on one hydrogen away from H2CO3, this K1 is small, yielding HCO32-. The subsequent K2 dissociation reaction to CO32- is relatively larger. Both reactions are nearly instantly reversible. But the hydration reaction CO2 + H20 <-> H2CO3 is so fast it is unmeasurable. Sampling changes the measurement. Unfortunately many textbooks merge unreacted aqueous CO2 gas and H2CO2 into a single hypothetical entity, which has apparently led to misunderstanding.
There is a highly abundant (>90%) reservoir of these readily available carbonate ions in seawater surface thin layer and well mixed layer which are not unreacted aqueous CO2 gas but which are very rapidly convertible to unreacted aqueous CO2 gas when the unreacted aqueous CO2 gas in seawater is depleted. And vice versa. We have shown this in Bromley & Tamarkin (2022). Depletion of CO2 in air as well as CO2 in water occurs by many different processes, and addition of CO2 to air and water occurs by many different processes. For example, outgassing of CO2 from water to air occurs to rebalance to Henry’s Law coefficient when CO2 concentration in air is being depleted by photosynthesis of land plants or by surface winds; conversely when aquatic plants like plankton bloom in ocean and absorb aqueous CO2 gas for their photosynthesis, then CO2 gas will be absorbed into water from air at higher ratio than is being emitted from water to air until the Henry’s Law coefficient is achieved for the water surface temperature. These opposing processes can and do occur simultaneously, which is probably a reason climatologist rarely if ever attempt to model Henry’s Law.
To summarize so far, when the approximately 1% of aqueous CO2 gas is either increased or decreased by any amount, due to any source or sink, this perturbation causes a recovery (following le Chatelier’s Principle) to rapidly return to that 1% by the using ocean’s chemistry systems, and there is an abundant reservoir of ionic carbonates in ocean water to achieve that recovery. In other words, the amount of CO2 emitted by humans replaces naturally emitted CO2 in the phase-state equilibrium, rather than accumulating in the atmosphere in addition to naturally occurring CO2. There is no CO2 accumulation in atmosphere. The ~1% can be perturbed in both directions by changes in temperature, salinity, pH, alkalinity, and by changes in partial pressure of the CO2 in the water or in the air. Adsorption and emission of CO2 are simultaneous and continuous in both directions at normal earth temperatures since CO2 molecules are continuously colliding with the surface and being emitted from the surface. The rate of absorption vs emission is the Henry’s Law coefficient for the local water temperature.
It is very important to mention that CO2 gas molecules and the three forms of carbonate ions (H2CO3, HCO32-, CO32-) do not need to migrate in water more than molecular distances to react with each other. Scientific publications which are adopted into the anthropogenic global warming (AGW) orthodoxy and climate models, for example work by (1) Bert Bolin (1960) first administrator of UN IPCC, and (2) Broecker and Peng (1974), and others, considered the thickness of the water thin layer and limited their calculus of aqueous CO2 migration to thickness of the water matrix. They define the chemical pathways that convert CO2 to HCO3– and vice versa as “chemical enhancement.” They calculate based on the thickness of the thin layer and migration time of the carbon species in the ocean matrix, however they ignore an equally critical variable which is the surface area of the gas – liquid interface, omitting more than 100 million square miles of air-water surface interface. This appears to be a major mistake in the AGW orthodoxy and modeling. Keep in mind that in these years of early papers seminal to IPCC orthodoxy, CO2 emissions were said to cause global cooling; I speculate they were focused on their modeling methods rather than empirical observations.
For example, “The rate limiting step for removal of anthropogenic CO2 from the air is vertical mixing within the sea rather than transfer across the air-sea interface.”(2) This statement conflicts with Fick’s 1st Law of net flux of gases.
Why did Bolin and Broecker & Pang (for example) consider only thickness of the ocean thin layer and not the huge square surface area of ocean? It is a puzzle for me. The 3 carbonate ionic species are distributed throughout the area of the thin layer as well as the well mixed layer beneath it and they surround the uncharged aqueous CO2 gas molecules in the thin layer and well mixed layer in a ratio of over 9:1. If the uncharged aqueous CO2 gas molecule is removed from the liquid matrix by either outgassing or by absorption into aquatic plants, then the carbonate reactions reverse and produce more uncharged aqueous CO2 gas molecules. And vice versa. And when the carbonate ion CO32- reacts with for example a very abundant ion such as calcium Ca2+ and solidifies, then the carbonate ion in water is thus reduced one for one. CO32- +Ca2+ = CaCO3 . Then as a consequence of this reaction (which occurs in warmer water near the surface, and reverses in colder deeper, higher pressure water), the prior carbonate and the hydration reactions are forced to re-equilibrate by absorbing more CO2 from air. Thus, there is a perpetual CO2 gas sink rate absorbing CO2 from air even while warming SST is slowly increasing outgassing CO2. These simultaneously opposed dynamic processes are mediated by Henry’s Law and the carbonate reactions.
Attached is a pdf (reference 3) which documents one of several gas chromatographic (GC) methods to quantify Henry’s Law coefficients. To use this method for CO2, since CO2 does not burn in the flame ionization detector (FID) flame, instead the effluent from GC column is passed first over a rubidium catalyst which stoichiometrically converts the CO2 to CH4, and then the effluent is directed onward to the FID where the CH4 is ionized and quantified. Alternatively, the FID can be replaced by a mass spectrometer and no catalyst is needed. I am pointing out that there is no mystery in Henry’s Law, there are many of these methods well documented by theory and experiment, but AGW proponents either ignore it or fail to understand it or both.
- Bolin, B., 1960. On the Exchange of Carbon Dioxide between the Atmosphere and the Sea. https://onlinelibrary.wiley.com/doi/pdf/10.1111/j.2153-3490.1960.tb01311.x Attached pdf.
- Broecker, W.S., and Peng, T.H., 1973. Gas Exchange rates between air and sea. https://onlinelibrary.wiley.com/doi/pdf/10.1111/j.2153-3490.1974.tb01948.x Attached pdf.
- Lee, S-H. et al, A Laboratory Experiment To Measure Henry’s Law Constants of Volatile Organic Compounds with a Bubble Column and a Gas Chromatography Flame Ionization Detector (GC-FID).(attached pdf, copyrighted.)
- Sander, R. Compilation of Henry’s law constants (version 4.0) for water as solvent. https://acp.copernicus.org/articles/15/4399/2015/
- NIST Chemistry WebBook, SRD 69, for Carbon Dioxide. https://webbook.nist.gov/cgi/inchi?ID=C124389&Mask=10#Solubility
- Dickson, A.G, et al. Andrew G. Dickson, Scripps Institution of Oceanography, UC San Diego, INTRODUCTION TO CO2 CHEMISTRY IN SEA WATER. https://www.iaea.org/sites/default/files/18/07/oa-chemistry-dickson-050916.pdf (attached)

Pingback: Alexanderhugget bevisas enkelt av Henrys Naturlag | KLIMATSANS
Thank you for your comments Mr. Hugget. My compliments too. I have a suggestion as a point of clarity for your consideration. For example, in this paragraph “Människan utsläpp utgör c:a 11 Gt C, gigaton kol. FN:s klimatpanel uppskattar den naturliga omsättningen av CO₂, mellan varma och kalla hav till 30 ggr så mycket och biosfärens omsättning till 40 ggr. Således är de naturliga utsläppen c:a 70 ggr så stora som våra utsläpp. Dessa utgör bara 1,4 % av alla utsläpp” allow me to suggest to keep your units the same. Instead of 11 Gt C, it would be clearer to the reader of your blog if you used Gt of CO2 throughout your post and adjust your calculations in line with that. Obviously the mass of carbon and carbon dioxide are not the same.
Att räkna molekyler eller volymer (Counting molecules or volumes). This is a very important point. Henry’s Law formula can be rearranged for several different purposes and all are correct and in continuous use since the 1800’s. Getting the units right for the different formulas and uses must be done with great care. How to decide which version or formula of Henry’s Law to use in the specific case of CO2 and ocean? I have decided to use the Henry’s Law version which is consistent with the NOAA Global Monitoring Lab (GML) at Mauna Loa because that lab is generally accepted as the defacto gold standard for CO2 measurements. GML reports CO2 as micromoles of CO2 per mole of dried air, which is equal to ppm. (Their reasons and methods are explained here: https://gml.noaa.gov/ccgg/about/co2_measurements.html )
In this case, ppm and ppmv are not equivalent and they cannot be converted because GML has changed the air samples by freezing out the water vapor and water, and we do not have the partial pressure nor concentration of the water and water vapor which was removed from each sample, which could enable a conversion to a volumetric unit such as micromoles per liter, which is an example of ppmv. The dimensionless version of Henry’s Law is consistent with the gold standard measurement of CO2 in air, which is TkH = c(aq)/c(g) where T is temperature in Kelvin. kH is the Henry’s Law constant. c(aq)/c(g) is the molar fraction of the gas in aqueous phase divided by the molar fraction of the gas in the gas phase above the liquid surface.
Similarly the unit ppm could be correctly defined based on mass instead of mole, as micrograms of CO2 per kilogram of air. Again this definition of ppm is inconsistent with GML’s definition and many decades of diligent measurements. Although GML’s micromoles of CO2 and moles of dried air can be easily converted to grams, but estimating the mass of the dried air introduces a large source of variance and error because the concentration of the gases in samples have been changed by removal of water and water vapor.
I hope these examples may help you and further your interest in correcting the misunderstandings in the climate change political agenda.
Again, good work! Thanks for reading and thanks for commenting.
LikeLike
Pingback: How Henry’s Law controls CO2 | budbromley